Toxoplasmosis
The Heaslip lab is focused on understanding the biology of human pathogen Toxoplasma gondii, an obligate intracellular protozoan parasite and the causative agent of Toxoplasmosis. T. gondii is transmitted to humans through ingestion of infective oocysts from gardens, or litter boxes in which infected cats have defecated or consumption of raw or undercooked infected meat. Infections are often asymptomatic in healthy individuals, but 1 in 5 people are chronically infected with the parasite. T. gondii causes life-threatening disease in immunocompromised persons, usually through reactivation of a chronic infection. It is also the leading cause of congenital neurological defects if acute infection occurs during pregnancy.
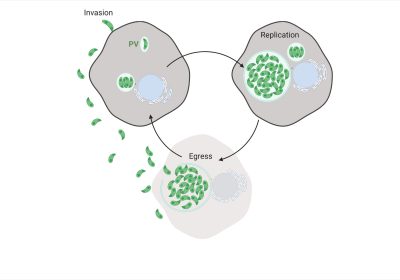
The T. gondii Lytic Cycle
An acute T. gondii infection is caused primarily by the parasites lytic cycle (Fig. 1A) involving: (1) invasion into the host cell, (2) replication within a specialized vacuole termed the parasitophorous vacuole (PV) and (3) host cell egress and dissemination, which results in the destruction of the infected cell. Invasion and egress depend on secretion of proteins from specialized secretory organelles called the micronemes and rhoptries, while intracellular survival requires secretion of proteins from the dense granules. Protein trafficking to the secretory organelles must be tightly controlled to ensure that the correct complement of proteins is delivered to each secretory organelle. We use a combination of parasite genetics, live cell imaging and single molecule biophysics to address how the actin cytoskeleton and associated myosin motors controls vesicular trafficking and organelle positioning in T. gondii.Mechanisms of Dense Granule Transport
Parasite survival and hence disease pathogenesis rely on its ability to secrete proteins from specialized secretory vesicles, named dense granules, into the host. Despite the importance of the dense granules, nothing was known about how the granules are transported form their site of synthesis at the Golgi to release sites at the parasite plasma membrane. In mammalian cells and yeast, the process of vesicle transport has been well characterized and involves the transport of secretory vesicles from by kinesin, dynein or myosin motors on their respective microtubule and actin tracks. Toxoplasma gondii, however, has a highly polarized organization which differs significantly from other eukaryotes – microtubules are only present at the parasite periphery and thus likely not involved in transport from the Golgi which is located at the center of the cell.
Using a combination of parasite genetics, live cell imaging and single particle tracking our lab has recently elucidated the molecular mechanisms of dense granule transport and surprisingly demonstrated that granule transport relies on filamentous actin and an unconventional myosin, TgMyoF. This data was represented a paradigm shift for the role of actin in one of the parasite’s most important cellular processes (Heaslip et al 2016; MBoC).
Our current work focused on further elucidating the mechanisms of dense granule transport with a focus on TgMyoF. TgMyoF is an essential protein, unique to Apicomplexan parasites and thus a potential target for parasitic drug development.
Dense granule motion in T. gondii